Context: R/R FL is associated with a poor prognosis and remains incurable; thus, better treatment options are needed. Epcoritamab is a subcutaneously administered CD3xCD20 bispecific antibody that has shown substantial antitumor activity in R/R FL. Objective: Evaluate safety and efficacy of epcoritamab with R2 in patients with R/R FL in arm 2 of a phase 1/2 open-label trial (EPCORE NHL-2; NCT04663347). Patients: Adults with R/R CD20+ FL were included. As of December 1, 2021, 30 patients (median age, 68 y) had enrolled. Interventions: Patients received subcutaneous epcoritamab (QW, cycle [C] 1–3; Q2W, C4–9; Q4W, C≥10 up to 2 y) + R2 for 12 cycles of 28 d. Step-up dosing and corticosteroid prophylaxis were required. Results: Of the 30 patients (epcoritamab 24 mg, n=3; 48 mg, n=27), 21 (70%) had stage IV disease and 20 (67%) had FLIPI scores 3–5. Median (range) number of prior lines of therapy was 1 (1–5), 30% had primary refractory disease, and 40% had disease progression within 24 mo after starting first-line treatment. At a median (range) follow-up of 5.1 mo (0.8–12.3), 25 patients (83%) remained on treatment; 5 patients discontinued treatment due to progression (n=2), AEs (n=2), or consent withdrawal (n=1). Common treatment-emergent AEs (TEAEs) of any grade (G) included infections (57%), injection-site reactions (50%), constipation (37%), fatigue (37%), and neutropenia (37%). CRS events occurred in 15 patients (50%; G1–2 43%, G3 7%), primarily in C1. All CRS events resolved; 3 patients were treated with tocilizumab, and 1 patient discontinued treatment due to CRS. One patient experienced G2 ICANS. No fatal TEAEs occurred. Overall response rate for the 27 evaluable patients was 100%; 93% had a complete metabolic response (CMR) and 7% had a partial metabolic response by PET-CT. As of the data cut, the longest duration of response was 7.0+ mo and ongoing. Conclusions: Subcutaneous epcoritamab + R2 exhibits promising efficacy, including a high CMR rate, in patients with R/R FL. The safety profile was consistent with prior data. Updated data will be presented. Funding: This study was funded by Genmab A/S and AbbVie.
Windows en Linux
Houdt de toets ctrl ingedrukt en draai het muiswiel omhoog of omlaag
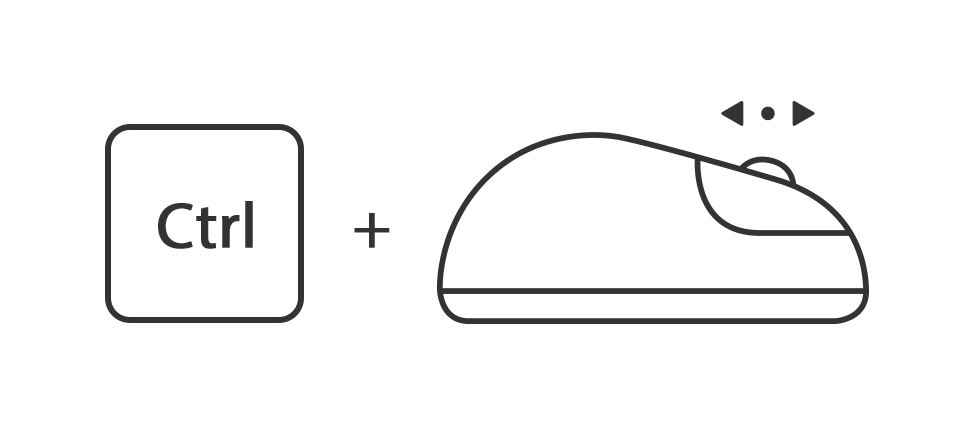
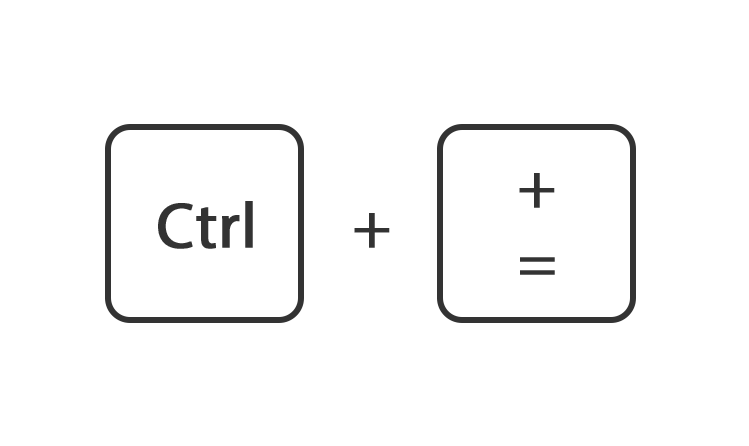
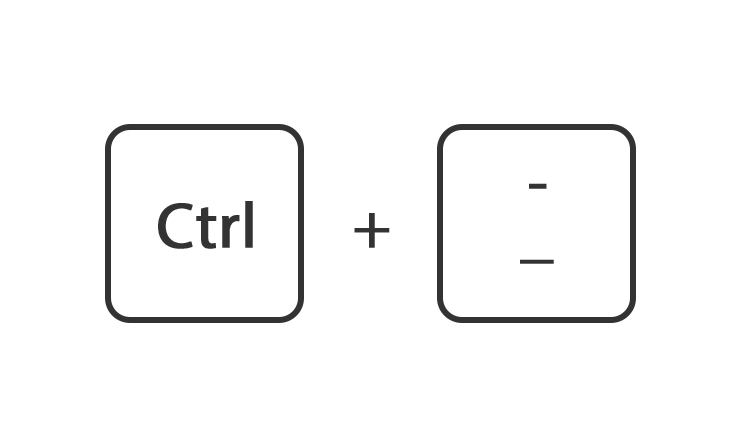
Of houdt de toets ctrl ingedrukt en druk tegelijk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom
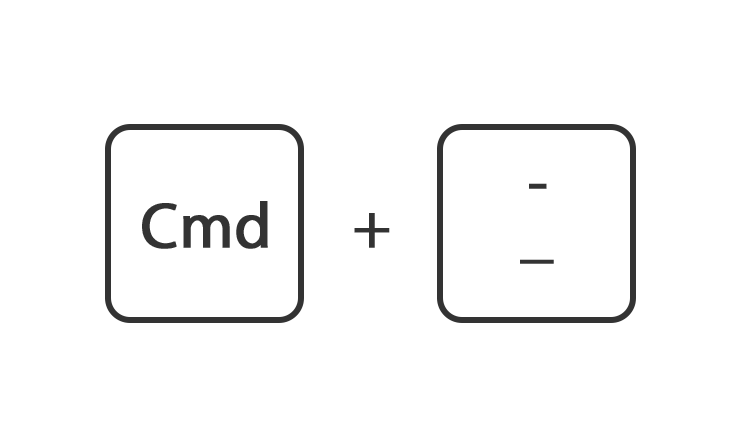
Mac
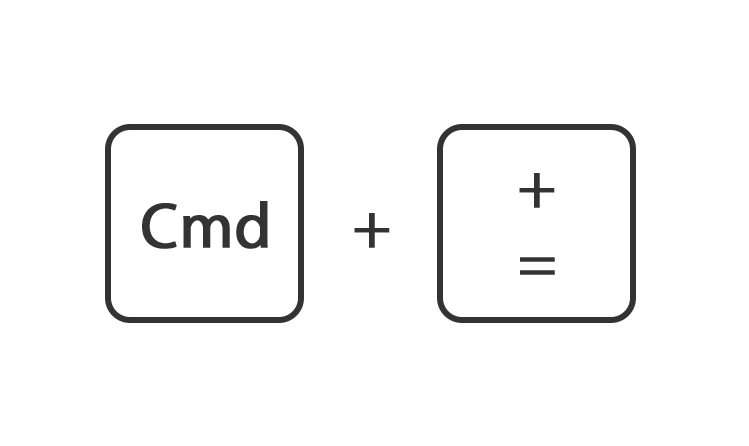
Houdt de toets command ingedrukt en druk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom.