Background: Frailty in newly-diagnosed multiple myeloma (NDMM) patients is associated with treatment-related toxicity, which negatively affects health-related quality of life (HRQoL). Currently, data on changes in HRQoL of frail and intermediate-fit MM patients during active treatment and post-treatment follow-up are absent. Methods: The HOVON123 study (NTR4244) was a phase II trial in which NDMM patients ≥ 75 years were treated with nine dose-adjusted cycles of Melphalan-Prednisone-Bortezomib (MPV). Two HRQoL instruments (EORTC QLQ-C30 and -MY20) were obtained before start of treatment, after 3 and 9 months of treatment and 6 and 12 months after treatment for patients who did not yet start second-line treatment. HRQoL changes and/or differences in frail and intermediate-fit patients (IMWG frailty score) were reported only when both statistically significant (p < 0.005) and clinically relevant (>MID). Results: 137 frail and 71 intermediate-fit patients were included in the analysis. Compliance was high and comparable in both groups. At baseline, frail patients reported lower global health status, lower physical functioning scores and more fatigue and pain compared to intermediate-fit patients. Both groups improved in global health status and future perspective; polyneuropathy complaints worsened over time. Frail patients improved over time in physical functioning, fatigue and pain. Improvement in global health status occurred earlier than in intermediate-fit patients. Conclusion: HRQoL improved during anti-myeloma treatment in both intermediate-fit and frail MM patients. In frail patients, improvement occurred faster and, in more domains, which was retained during follow-up. This implies that physicians should not withhold safe and effective therapies from frail patients in fear of HRQoL deterioration.
Windows en Linux
Houdt de toets ctrl ingedrukt en draai het muiswiel omhoog of omlaag
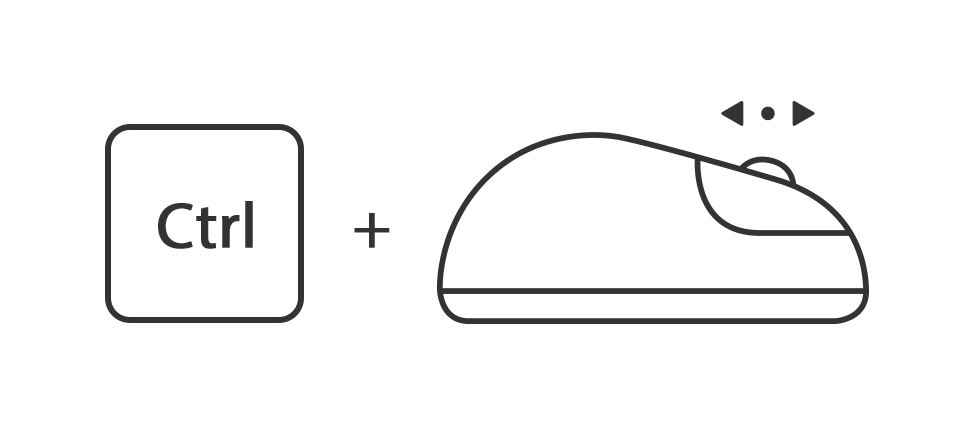
Of houdt de toets ctrl ingedrukt en druk tegelijk op de toets + of -
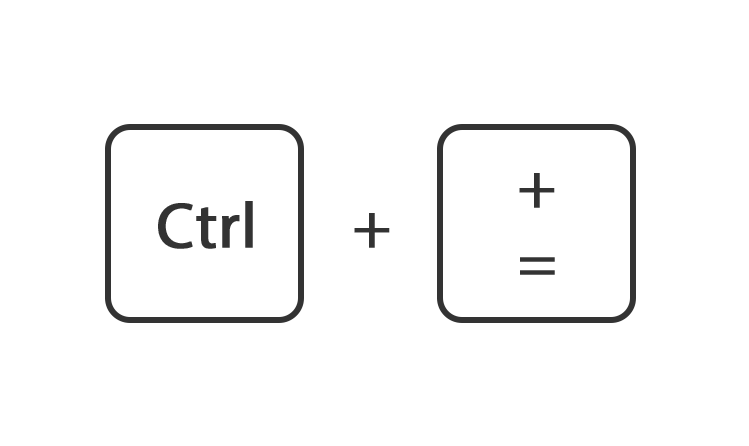
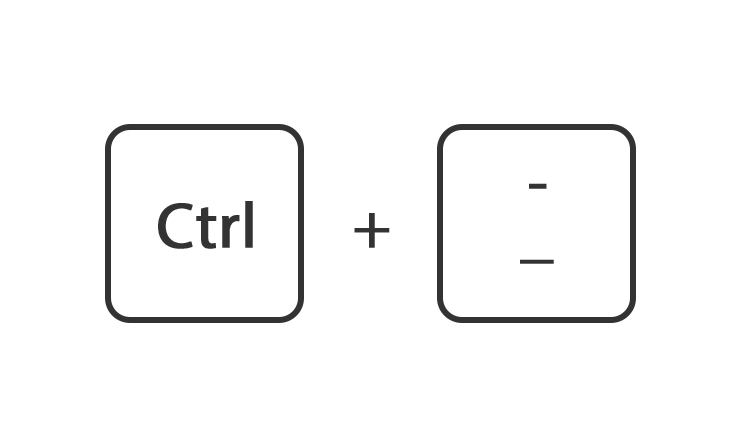
Of ga rechtsboven naar instellingen en gebruik de instelling zoom
Mac
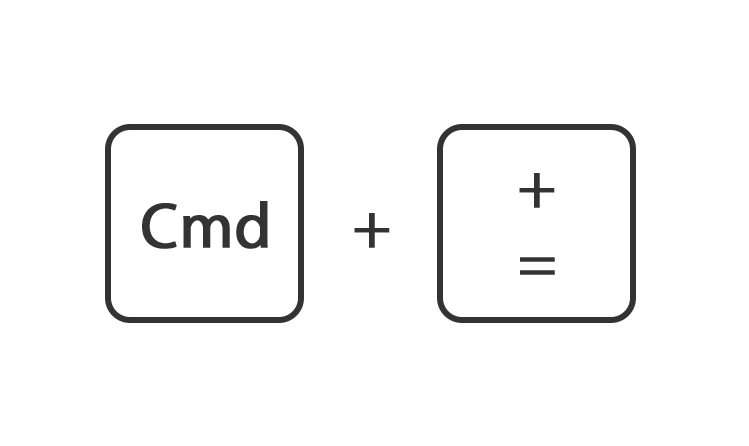
Houdt de toets command ingedrukt en druk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom.