Context: CARTITUDE-2 (NCT04133636) Cohort B is evaluating cilta-cel in patients with MM and early relapse after initial therapy. These patients have functionally high-risk disease and unmet medical needs, as early relapse post-ASCT is associated with a poor prognosis. Objective: To present updated results from CARTITUDE-2 Cohort B. Design: Phase 2, multicohort study. Patients: Eligible patients had MM, 1 prior LOT (PI and IMiD required), disease progression per IMWG, and no previous treatment with CAR-T/anti-BCMA therapies. Intervention: Single cilta-cel infusion (target dose 0.75×106 CAR+ viable T-cells/kg) post lymphodepletion. Main Outcome Measures: Safety and efficacy were evaluated. Primary endpoint was MRD negativity at 10-5. Management strategies were used to reduce the risk of movement/neurocognitive AEs (MNTs). Assessments included pharmacokinetics (PK) (Cmax, Tmax of CAR+ T-cell transgene levels in blood), CRS-related cytokine (eg, IL-6) levels over time, peak cytokine levels by response and CRS, association of cytokine levels with ICANS, and CAR+ T-cell CD4/CD8 ratio by response, CRS, and ICANS. Results: As of January 2022 (median follow-up 13.4 months), 19 patients (median age 58.0 years; 74% male; 79% with prior ASCT) received cilta-cel. ORR was 100% (90% ≥CR and 95% ≥VGPR). Median time to first response was 0.95 months and median time to best response was 5.1 months. Median DOR was not reached. Of 15 MRD-evaluable patients, 14 (93%) achieved MRD 10-5 negativity. At 12 months, the event-free rate was 88.9% and PFS rate was 90%. CRS occurred in 16 (84.2%) patients (1 gr 4); median time to onset was 8 days. CRS resolved in all patients. 1 patient had ICANS (gr 1); 1 patient had MNT (gr 3; previously reported). 1 death occurred post-cilta-cel due to progressive disease (day 158). Preliminary PK data showed CAR-T cell peak expansion on day 13.1 and median persistence was 76.9 days. Conclusions: A single cilta-cel infusion resulted in deep and durable responses and manageable safety in functionally high-risk patients with MM and early clinical relapse/treatment failure to initial therapy. We will present updated and detailed PK/cytokine/CAR-T subset analyses and clinical correlations to provide novel insights into biological correlates of efficacy/safety in this population.
Windows en Linux
Houdt de toets ctrl ingedrukt en draai het muiswiel omhoog of omlaag
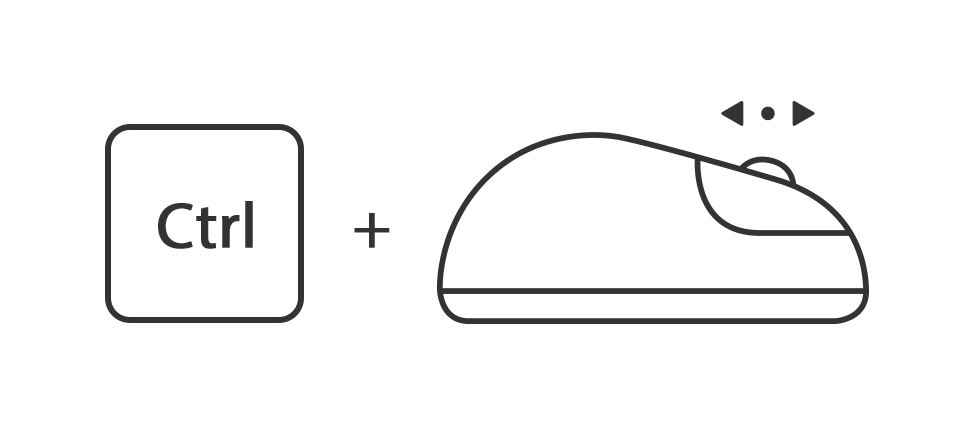
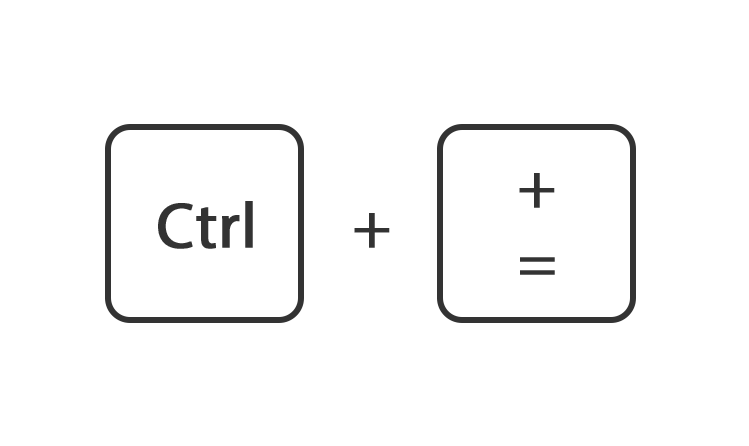
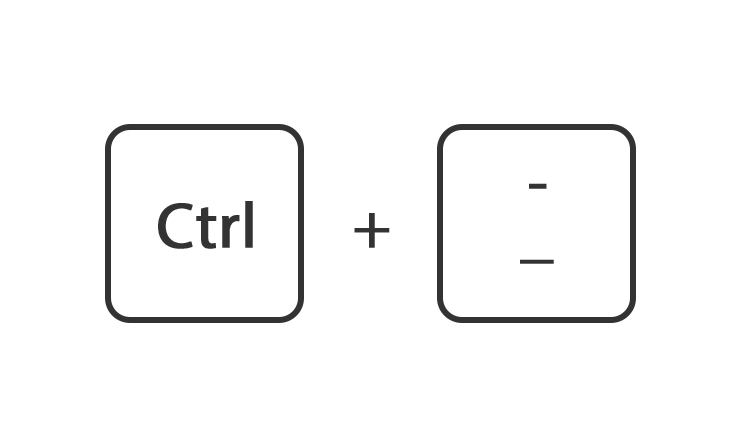
Of houdt de toets ctrl ingedrukt en druk tegelijk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom
Mac
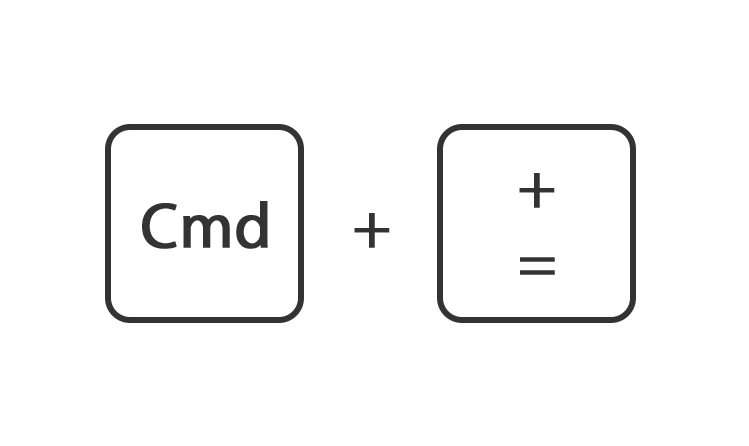
Houdt de toets command ingedrukt en druk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom.