Peripheral T-cell lymphomas (PTCL) comprise a heterogeneous group of mature T-cell neoplasms with an unfavorable prognosis; presentation with stage I(E) disease is uncommon. In clinical practice, an abbreviated chemotherapy treatment regimen combined with radiotherapy (combined modality treatment (CMT)) is commonly used, although evidence from clinical trials is lacking. The aim of this nationwide population-based cohort study is to describe first-line treatment and outcome of patients with stage I(E) PTCL. All newly diagnosed patients ≥ 18 years with stage I(E) anaplastic large cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL) and peripheral T-cell lymphoma NOS (PTCL NOS) in 1989-2020 were identified in the Netherlands Cancer Registry. Patients were categorized according to treatment regimen, i.e. chemotherapy (CT), radiotherapy (RT), CMT, other therapy and no treatment. The primary endpoint was overall survival (OS). Patients with stage I(E) ALCL, AITL and PTCL NOS (n=576) were most commonly treated with CMT (28%) or CT (29%), 2% underwent SCT. RT only was given in 18%, and 8% received other therapy and 16% no treatment. Overall, the 5-year OS was 59%. According to subtype, 5-year OS was superior for ALCL as compared to PTCL NOS and AITL (68% vs. 55% and 52%, respectively; p=0.03). For patients treated with CMT, 5-year OS was significantly higher (72%) as compared to patients treated with either CT or RT alone (55% and 55%, respectively; p.
Windows en Linux
Houdt de toets ctrl ingedrukt en draai het muiswiel omhoog of omlaag
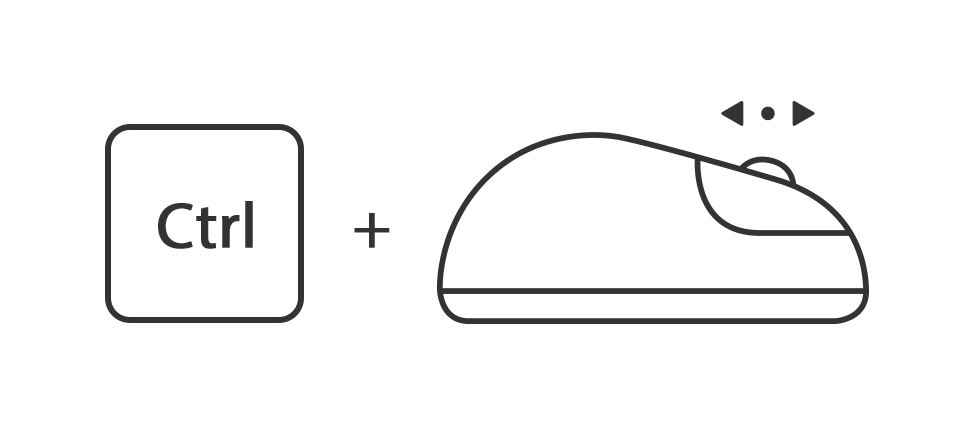
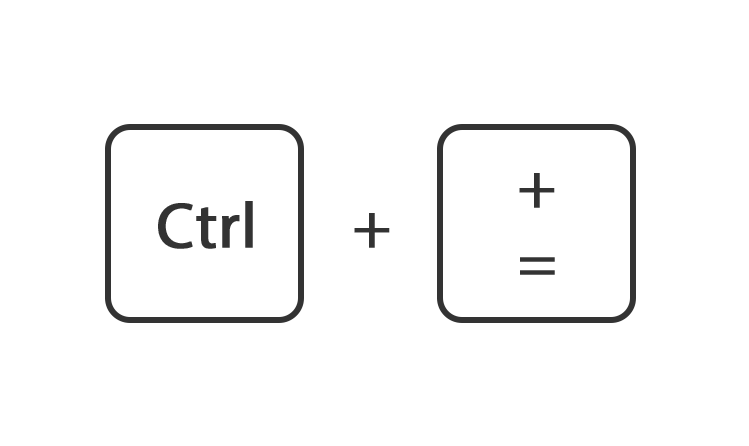
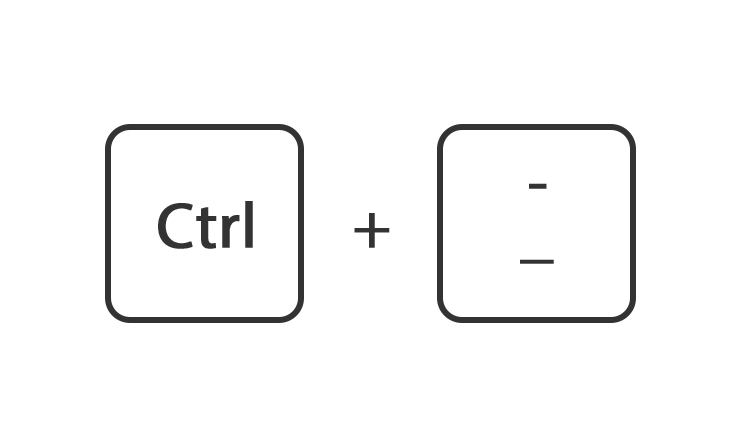
Of houdt de toets ctrl ingedrukt en druk tegelijk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom
Mac
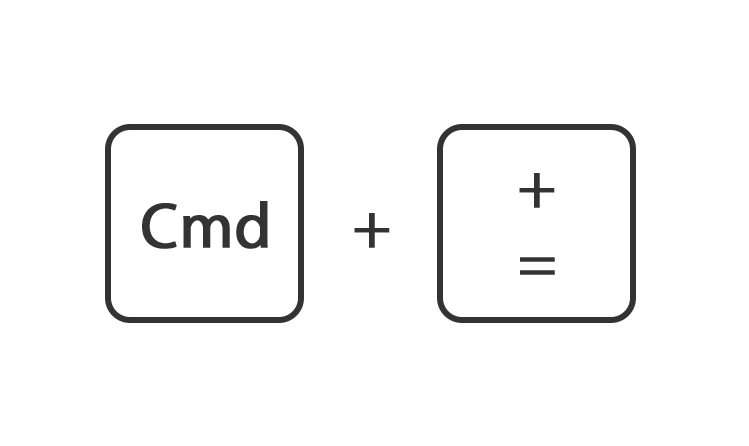
Houdt de toets command ingedrukt en druk op de toets + of -
Of ga rechtsboven naar instellingen en gebruik de instelling zoom.